Identification and Potential of Diabetes Stem Cells
Diabetes is a chronically progressing disease that does not heal naturally. It is believed to be caused by factors such as genetics, lifestyle, and autoimmunity, but the exact causes are still unknown.
Our research team discovered abnormal cells in hematopoietic stem cell fractions that cause diabetes and its complications, and exhibit refractory characteristics. These abnormal cells persist even when blood sugar levels normalize and maintain their properties as disease stem cells. We named these cells “Diabetes Stem Cells.”
Furthermore, the possibility of curing diabetes and its complications by eliminating these Diabetes Stem Cells has been demonstrated.
Encounter with Diabetes Stem Cells
Discovery of Strange Cells and a New Approach to Diabetes Treatment
In 1980, John Najarian and David Sutherland at the University of Minnesota developed a method for treating chronic pancreatitis by performing a total pancreatectomy and subsequently transplanting islets into the patient’s own liver. This method prevented diabetes resulting from the pancreatectomy and improved diabetes in patients already suffering from the disease. This method later evolved into islet transplantation therapy used to treat type 1 diabetes.
The liver, with its high regenerative capacity and similar embryological origin to the pancreas, is an ideal organ for islet regeneration. In October 1999, the author planned a gene therapy targeting the liver at Baylor College of Medicine, based on islet transplantation. Using transcription factors involved in pancreatic development (Pdx-1, Ngn3, NeuroD1), islets were regenerated in the liver of mice, aiming for a cure for diabetes.
However, Pdx-1 produced not only insulin-producing cells but also exocrine pancreatic cells in the liver, causing severe hepatitis. In 2001, the expression vector for Ngn3 was created, but tropical storm Allison flooded the Texas Medical Center, destroying all samples in the ultra-low temperature freezers. Miraculously, only the NeuroD1 vector was saved, and by the end of 2002, the paper “NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice” (Nature Med, 2023) was completed.
This research successfully recreated islets in the liver using NeuroD1. However, one unresolved question remained. Strange cells producing proinsulin were found in the liver of untreated diabetic mice used as controls for gene therapy. These cells were located right next to the portal vein capillaries in the liver of hyperglycemic mice. In 2003, a paper including microscopic images of these strange proinsulin-positive cells was accepted.
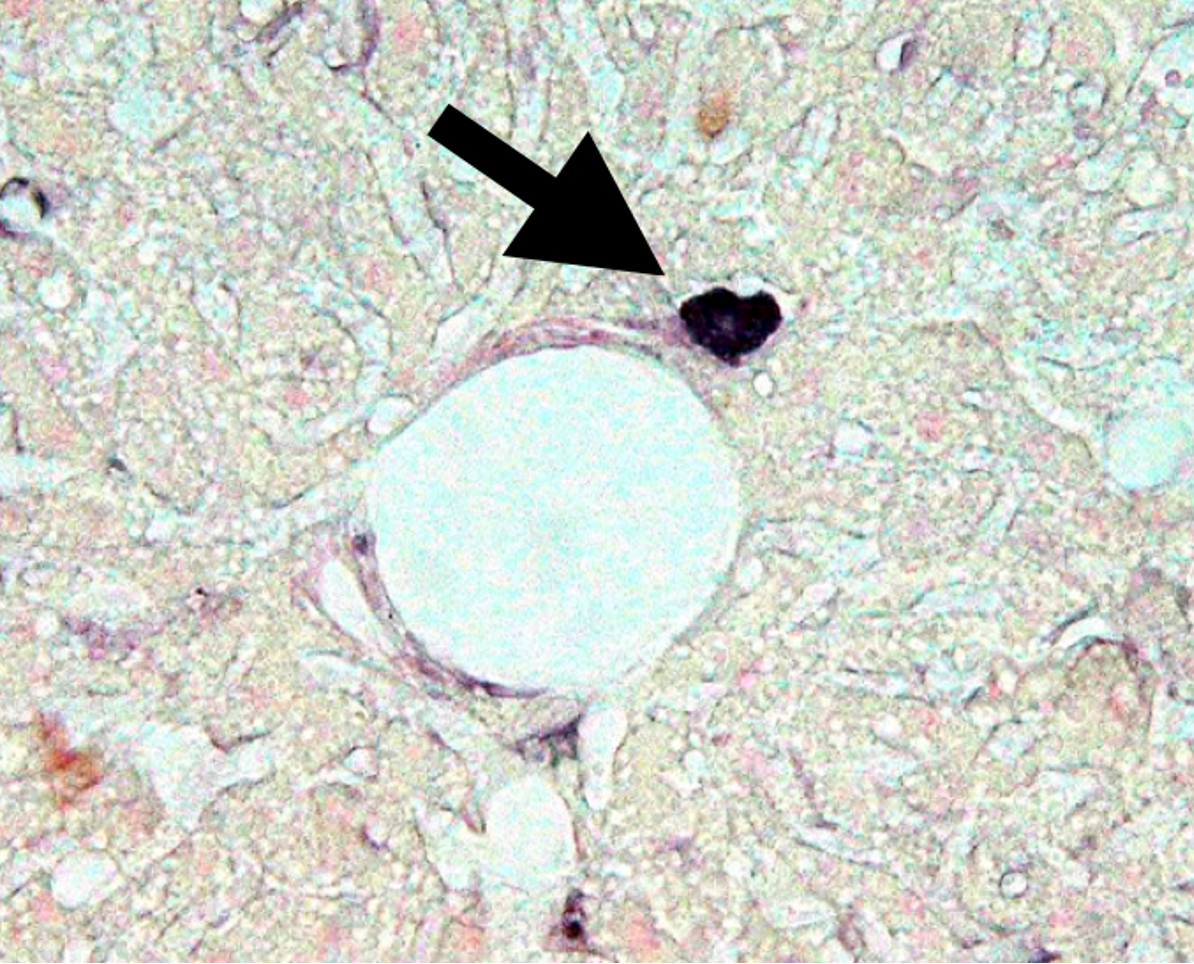
Abnormal Cells Producing Proinsulin
Challenge of Strange Cells in Diabetes Treatment
Through our attempts to regenerate islets in the liver, we discovered abnormal cells producing proinsulin. These cells express Pdx-1 and appear to be precursor cells of islets, but they produce proinsulin, a precursor of insulin, with no effect on lowering blood sugar. Identifying these cells is a crucial task for human application.
Despite many years of diabetes research, no one could find these strange cells. It is essential to understand why these cells appear and their purpose. Research using various diabetes model animals revealed that these cells are derived from bone marrow, express both proinsulin and islet-related proteins, and produce TNF-α, an inflammatory cytokine. This results in insulin resistance in organs.
NeuroD1 gene therapy in the liver regenerated islets and improved hyperglycemia in STZ diabetic mice, but this is only a result in an experimental diabetes model. For application in human diabetes, methods to suppress TNF-α expression in these abnormal cells need to be found.
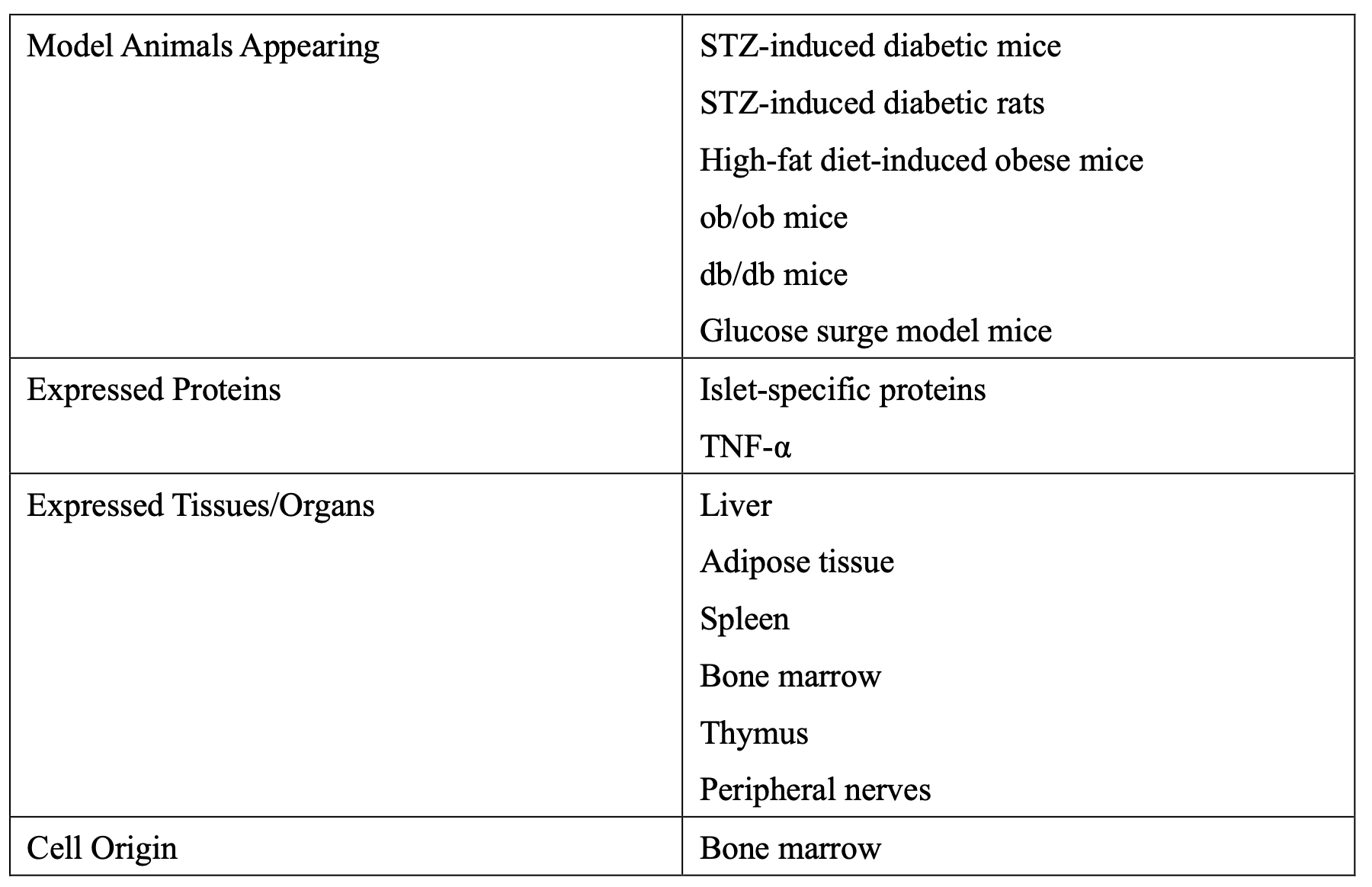
Discovery and Impact of Diabetes Stem Cells
The Mystery of Indelible Memory
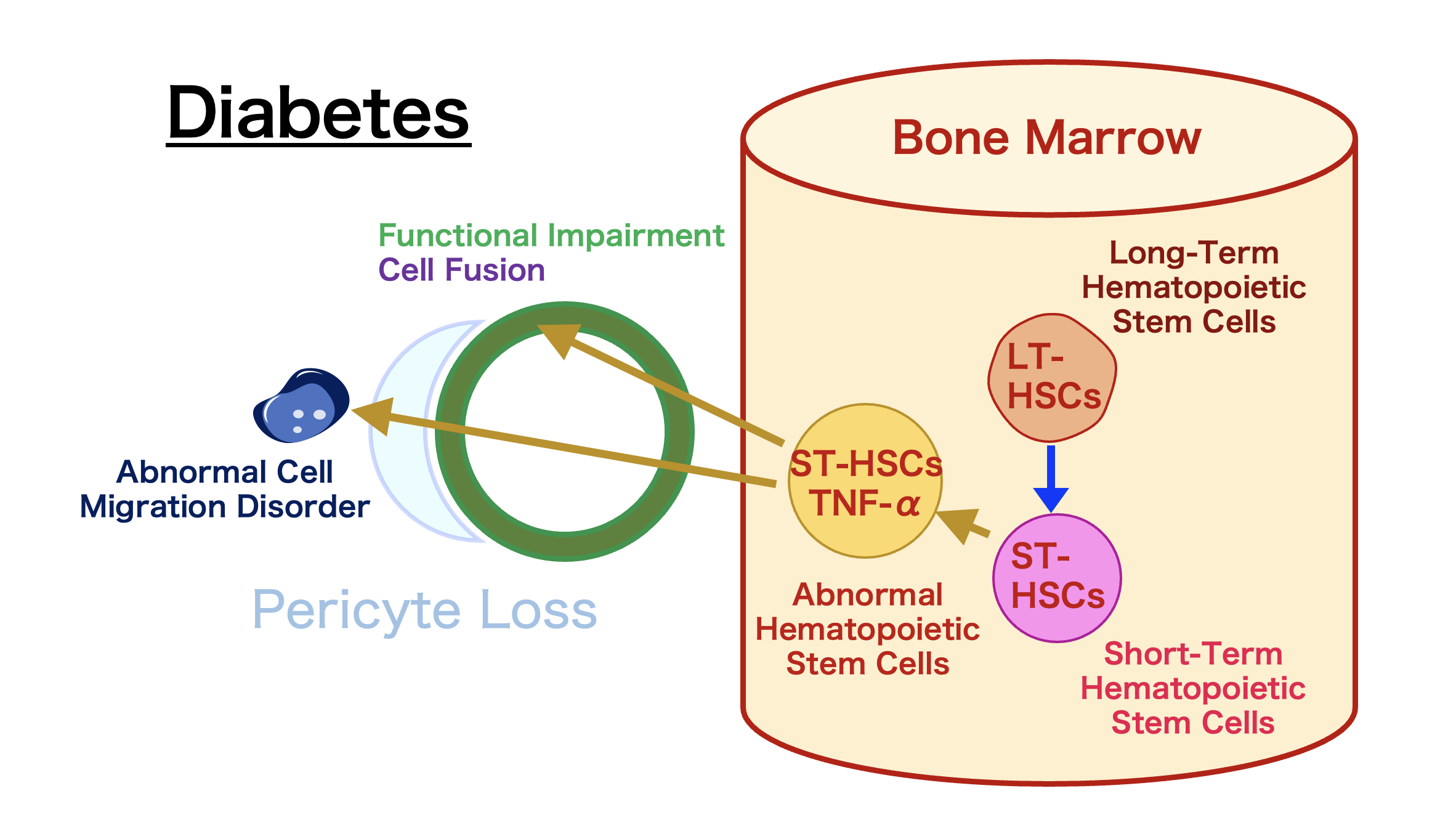
Further research revealed abnormal cells producing proinsulin and TNF-α in various organs. These bone marrow-derived cells showed abnormalities in Kupffer cells and stellate cells of the liver, ICC of Cajal in the intestines, microglia in the brain, osteoclasts in bones, adipocytes, tissue macrophages, and endothelial cells in the kidneys.
Investigation revealed that these abnormal cells originate from CD106-positive cells within short-term hematopoietic stem cells. The abnormality in these stem cells causes dysfunction in organs throughout the body. Normally, these cells regenerate damaged tissue, but their function is impaired by diabetes.
We call this phenomenon “Diabetes Stem Cells,” believing that these cells remain in the body as an “indelible memory” of diabetes, exacerbating the disease. Due to the abnormalities in stem cells, every instance of hyperglycemia increases the abnormal stem cells, leading to vascular damage and organ dysfunction.
Our bodies have three homeostasis maintenance systems: the nervous system, the immune system, and the endocrine system. We believe there is a fourth homeostasis system, the regenerative system, centered on long-term hematopoietic stem cells. Diabetes impairs this regenerative system, making it an “incurable disease.”
In an experimental model of diabetic neuropathy, removing the hyperglycemic trigger and eliminating pathogenic CD106-positive hematopoietic stem cells resulted in healing. This research indicates that eliminating “Diabetes Stem Cells” is key to diabetes treatment.
Discovery and Research of Diabetes Stem Cells
Abnormal cells were found in hematopoietic stem cell fractions that cause diabetes, which do not disappear even when b/s levels are normalized, creating refractory characteristics.
Discovered that diabetes is a disease caused by abnormalities in hematopoietic stem cells and developed a method to cure diabetes by isolating and eliminating “Diabetes Stem Cells.”
Cell Targeting Technology (Biozipcode)
Aiming to develop cell-targeted drugs as a new drug delivery method for the next generation, beyond the current era of molecular-targeted drugs.
Developing a completely new diabetes and diabetes complication cure using Biozipcode that causes no side effects.
Using cell targeting technology with Biozipcode to develop side-effect-free anticancer drugs, providing treatments that minimize patient burden.
Aiming to develop scaffold materials that promote natural wound healing, adhering to tissues without the need for transplantation or suturing, and enhancing quality of life (QOL).
Are You Interested in Research at the Department of Regenerative Medicine?
Are You Interested in Research at the Department of Regenerative Medicine?
You can send a direct inquiry to the Department of Regenerative Medicine through the contact form.